Table of Contents
Abstract
Objective: To investigate the association of prepregnancy and prenatal depression and/or anxiety on preterm birth (PTB), while also exploring Hispanic/Latina ethnicity as a potential effect modifier.
Methods: Study population included respondents of UT-PRAMS (2016–2019). Associations between prepregnancy and prenatal depression and/or anxiety and PTB were evaluated using Poisson regression models accounting for stratified survey sampling.
Results: Women with prepregnancy and prenatal depression and anxiety, compared to those without, had a 67 percent (95% CI: 19%, 134%) higher probability of experiencing PTB, after controlling for relevant sociodemographic, lifestyle, and reproductive history factors. Impact of depression on PTB was slightly higher than impact of anxiety. Hispanic/Latina ethnicity was found to protect against PTB for those with prepregnancy and prenatal depression alone (aPR: 0.53, 95% CI: 0.24, 1.21) or both depression and anxiety (aPR: 0.51, 95% CI: 0.18, 1.40) compared to being non-Hispanic/Latina (aPR: 1.79, 95% CI: 1.25, 2.55 for depression alone; aPR: 1.62, 95% CI: 1.18, 2.21 for depression and anxiety).
Conclusions: Overall, Utah women reporting prepregnancy and prenatal depression and anxiety were more likely to have a PTB. Being of Hispanic/Latina ethnicity was found to mitigate the risk of PTB among women with depression and anxiety.
Implications: Prepregnancy and prenatal mental health screenings and treatment are key to lessening the impacts of depression and anxiety on both mother and infant. Hispanic/Latina ethnicity may be protective against PTB among women experiencing mental distress. Whether this is through increased social support or through a different mechanism should be explored in future research.
Introduction
In 2019, the national preterm birth (PTB) rate rose for the fifth year in a row, affecting approximately 10 percent of infants and causing growing concern among medical and public health officials.2 PTB poses several risks to an infant including immature lungs, difficulty regulating body temperature, poor feeding, and slow weight gain.3 Maternal depression and anxiety have been directly linked to PTB, with long-term effects on both mother and baby.4-7 This is especially relevant since depression is the most common psychiatric disorder in the US and highest among women, with anxiety not far behind.9 A recent study reported that women with both mental health disorders were found to have a higher rate of PTB than those with only depression, only anxiety, or without either disorder.10 An extensive body of prior literature has established that a history of prepregnancy depression and anxiety is a strong risk factor for prenatal depression11-17 and that prepregnancy and prenatal depression and anxiety are highly comorbid.18,19 For that reason, this study explores the potential cumulative impact of prepregnancy and prenatal depression and anxiety (PPDA) on the likelihood of PTB in Utah. Ethnicity is also highly associated with the risk of PTB,20 and this study will look at what effect Hispanic/Latina ethnicity, which makes up the second-largest ethnic group in Utah, may have on risk for PTB.
Methods
Study Population: This is a cross-sectional study design using data from the Utah Pregnancy Risk Assessment Monitoring System (UT-PRAMS) survey, Phase 8 (January 1, 2016, through December 31, 2019). The Centers for Disease Control (CDC) developed the standardized data collection methodology used for the national-level PRAMS survey and continues to provide oversight for the survey’s methodology and protocol.21-23 PRAMS is a mixed-mode surveillance system (mail and telephone) that uses birth certificate information as its population-based sampling frame. One key aspect of PRAMS is the stratified systematic sampling, which oversamples on features related to high-risk women such as mothers of low-birthweight infants, living in high-risk geographic areas, and belonging to racial/ethnic minority groups.21
UT-PRAMS Phase 8 drew stratified (by maternal education and infant birthweight) samples of approximately 200 new mothers (2–6 months after delivery of a live birth) every month.24 New mothers were contacted via mailed questionnaire (available in English and Spanish) multiple times and telephone follow-up. An informed consent document was included with each survey packet explaining the participants’ rights. Consent is implied if the survey is completed. The total sample of Utah mothers completing the PRAMS Phase 8 questionnaire was 5814, reflecting an estimated population of 188,700 women. The sample comprises 16.2 percent Hispanic/Latinas and 10.9 percent non-White, making it representative of Utah’s race/ethnicity makeup consisting of Hispanic/Latinas (14.4%), Blacks or African Americans (1.5%), American Indians or Alaskan Natives (1.6%), Asians (2.7%), Native Hawaiians or Pacific Islanders (1.1%), or those of two or more races (2.2%).25 The expected national PRAMS response rate is 60%, with Utah exceeding this goal at 65% (2016), 66% (2017), 62% (2018), and 69.5% (2019).
Mothers’ responses were linked to extracted birth certificate data items, including pregnancy complications for index birth. The PRAMS weighting process produces an analysis weight considering the stratified sampling along with nonresponse and noncoverage components. The analysis weight of the PRAMS data can be interpreted as the number of women like herself in the population that each respondent represents. This study and the use of PRAMS data (de-identified) have been acknowledged by the University of Utah Institutional Review Board as nonhuman subject research.
Outcome: The primary outcome of interest was whether or not a woman experienced PTB, defined as a live birth before 37 weeks into the pregnancy.2 The obstetric estimation of gestation, which uses ultrasonography within the first two trimesters to determine gestational age and the estimated delivery date, is added to the birth certificate within 24–48 hours of the birth.26,27 However, it is important to note that the obstetric estimation of gestation may vary by up to 10–14 days.28
Exposure: The PRAMS questionnaire asks women whether they had experienced depression or anxiety (each requiring yes/no answers) during the 3 months before the most recent pregnancy (prepregnancy) or during the most recent pregnancy (prenatal). We created a combined impact variable to include women who had both depression and anxiety in the 3 months prior to conception and during pregnancy (PPDA). This combined impact variable was chosen due to the common comorbidity of these 2 disorders in women (both prepregnant and prenatal) as well as the possibility that the combination for a sustained period of time may result in a cumulative impact increasing the risk for PTB.
Covariates: Confounding factors believed to influence both depression and anxiety as well as PTB were determined based on prior literature. Demographic and lifestyle factors included race/ethnicity, maternal age, marital status, maternal education, total household income, and body mass index (BMI). Additionally, smoking, drinking, and prior history of high blood pressure and diabetes as well as reproductive history were also considered as potential confounding factors.7,20, 29-32 Given prior theories on the role that maternal race/ethnicity may play in mental health-adverse birth outcome associations,33 we additionally tested whether Hispanic/Latina ethnicity may modify the association between prepregnancy and prenatal depression and/or anxiety and PTB.
Statistical Analysis: Participant characteristics were reported by the exposure of interest (ie, PPDA) and took into account PRAMS’ weighted analysis formatting.19 All variables were dichotomous or categorical and reported as weighted percentages.
To explore the associations between prepregnancy and prenatal depression and/or anxiety and PTB, we used Poisson regression models with a robust error variance. The models, accounting for PRAMS’ use of stratified sampling, generated adjusted prevalence ratios (PR) and 95% confidence intervals (CI).34,35 The referent group was women without PPDA. Mothers who did not have data for key exposure variables were removed from the analysis population. Effect modification by Hispanic/Latina ethnicity was conducted via a stratified analysis and on a multiplicative scale using the Wald test. Stata 15.1 was used for the analysis.
Results
After excluding missing values from key exposure variables (depression before/during pregnancy and anxiety before/during pregnancy), 4166 women (72%) were included in the primary analyses, reflecting an estimated population of 136,090 women (Figure 1).

Among this study population, 13 percent had PPDA while 87 percent did not (Table 1). White non-Hispanic/Latina women were more likely to report PPDA (16%) compared to white Hispanic/Latina women (11%).


The median age was 28 years (mean age 28.8, SE 8.7). The majority of women were White (89.1%), non-Hispanic/Latina (83.8%), married (83.3%), had at least some college (72.9%), and did not smoke (91.5%) or drink alcohol (67.6%) within 2 years before pregnancy. A high percentage did not have the common comorbidities of diabetes (99.6%) or high blood pressure (98.8%) within 2 years prior to pregnancy. Household income ranged from $20,001 to $57,000 for 46.4 percent of the population and was above $57,001 for the remaining participants (Table 1).
Almost a third of mothers in this study gave birth for the first time (34.8%) while the others had experienced 1–3 (56.7%), 4–7 (8.2%), and 8+ (0.3%) previous live births, respectively. Of those with previous live births, 98.1% delivered a single child, 1.8% delivered twins, and 0.1% delivered multiples. Of women with a previous live birth 5% of women reported previous PTB. The prepregnancy BMI categories for participants was underweight (10.7%), normal weight (50.8%), overweight (13.5%), and obese (25%). Women with PPDA, compared to those without, were more likely to have a history of smoking and/or drinking alcohol within the past 2 years, to be obese, have high blood pressure, a history of PTB, and/or given birth to multiples.
Association Between Depression and/or Anxiety and Preterm Birth: In the unadjusted analysis we found that women reporting PPDA, compared to those without, were 1.55 (95% CI, 1.22, 1.99) times more likely to experience PTB. Those with depression alone were 1.48 (95% CI, 1.18, 1.85) times more likely to experience PTB, and those with anxiety alone were 1.44 (95% CI, 1.17, 1.78) times more likely (Table 2).
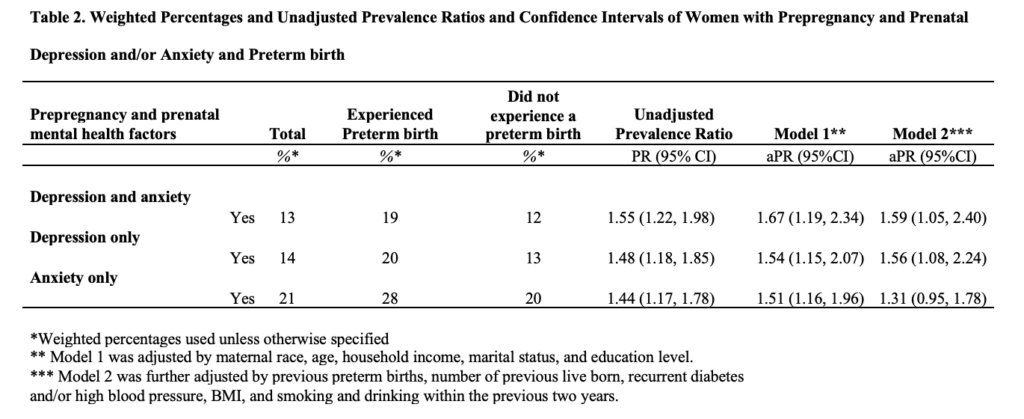
After adjusting for maternal race, age, household income, marital status, and education level, women with PPDA, compared to those without, were 1.67 (95% CI, 1.19, 2.34) more likely to have PTB. Those with depression were 1.54 (95% CI, 1.15, 2.07) times more likely to experience PTB, while those with anxiety were 1.51 (95% CI, 1.16, 1.96) times more likely (Table 2).
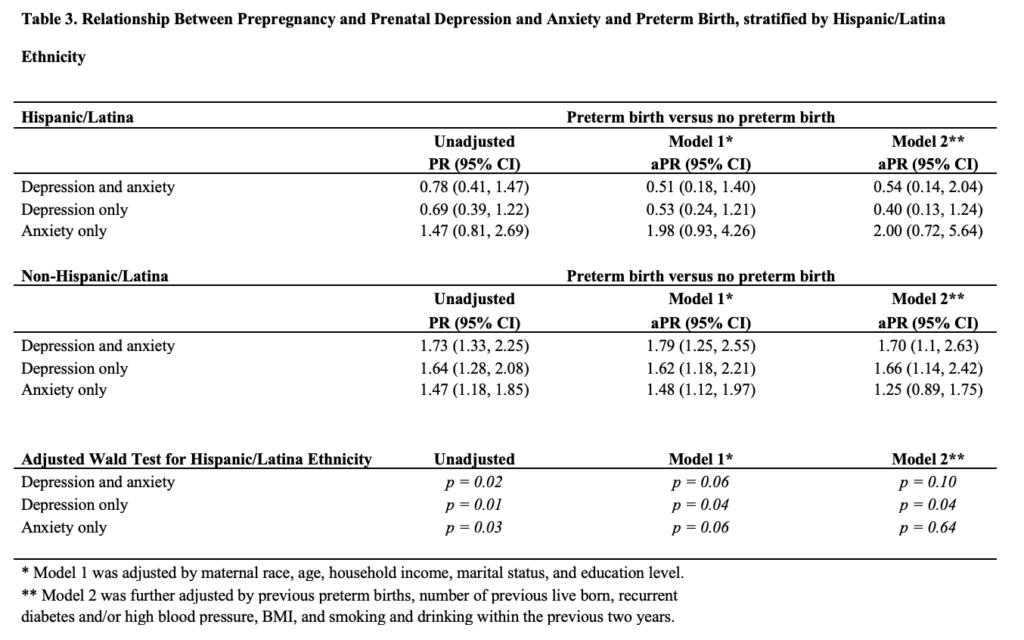
After additional adjustment for previous preterm births, number of previous live born, recurrent diabetes and/or high blood pressure, BMI, and smoking and drinking within the previous 2 years, those with PPDA were 1.59 (95% CI, 1.05, 2.40) more likely to have PTB, while those with depression were 1.56 (95% CI, 1.08, 2.24) more likely to experience PTB and those with anxiety 1.31 (95% CI, 0.95, 1.78) more likely (Table 2). Stratification by Hispanic/Latina ethnicity showed effect modification for prepregnancy and prenatal depression and PTB in both the parsimoniously adjusted model and fully adjusted model (Wald P for both=0.04) (Table 3).
Discussion
After accounting for relevant sociodemographic, lifestyle, and reproductive history factors, women with prepregnancy and prenatal depression and anxiety, compared to those without, had a 67 percent higher probability of experiencing PTB. Women experiencing depression but not anxiety had a 56 percent higher probability of experiencing PTB, and those with anxiety but not depression were 51 percent more likely to have PTB. Our findings indicate that among the entire study population, the cumulative effect of depression and anxiety has a greater potential impact on PTB risk than either alone. Effect modification by Hispanic/Latina ethnicity was found to act as a protective factor against PTB for those with prepregnancy and prenatal depression and anxiety as well as those with depression only.36
Strengths of the Study: The use of PRAMS data allows for a population-based analysis that is representative of all women in Utah, including at-risk women, due to its systematic stratified sampling scheme. Our ability to capture self-reported depression and anxiety data before and during pregnancy and to link these data to birth records for PTB assessment is novel, especially since questions on maternal depression and anxiety were added to the UT-PRAMS survey only in 2016.37 The UT-PRAMS questionnaire and linked birth records also allow us to take into account multiple important confounding factors including sociodemographic, lifestyle, health, and reproductive history.
Limitations of the Data: This study faces the limitation of selection bias by only focusing on mothers who had a live birth.38,39 The precision and accuracy of obstetric estimation of gestation, which can vary up to 14 days, may potentially lead to misclassification bias. Missing data for the outcome, key exposures, and covariates could lead to selection bias. Recall bias may also be a factor, as the questionnaire asks women at 2–6 months postpartum on experiences they had before and during pregnancy. Social desirability bias may also be a factor as not all mothers may wish to report on their smoking, drinking, or drug use due to the stigma associated with them. Additionally, the PRAMS questionnaire asks women to confirm that they experienced anxiety and/or depression before and during pregnancy without regard to standardized testing and clinical diagnoses.40 This may result in the data overestimating the prevalence of diagnosed depression and anxiety or underestimating based on poor screening.41 The severity of the mental disorder(s) is also not addressed. During PRAMS Phase 8, 43.8% of women were screened for depression during the 12 months before pregnancy, compared with 68.9% during prenatal care visits, and 85.9% during postpartum health care visits. This study did not explore the impact of stress and abuse factors on PTB among UT-PRAMS participants. The intensity of alcohol consumption and tobacco use are not provided in the data, which may directly impact the potential association between health behavior and the risk of PTB; these questions should be included in future questionnaires. The impact of prepregnancy and prenatal depression and/or anxiety on postpartum depression would also benefit from additional study.2, 42 Finally, we were not able to explore how race may modify the association between maternal depression and/or anxiety and PTB due to only having access to dichotomous race variable (White vs non-White). Given the high rate of PTB among Black women, more research is needed on potential factors that can explain this disparity.33
Interpretation: The findings of an association between prepregnancy and prenatal depression and anxiety and PTB is in harmony with a large body of prior research.2, 7, 25, 34-45 It is notable that many studies look at depression and anxiety separately instead of as ongoing comorbidities, and this has led to varying results in the literature.2,17,27 Adhikhari, et al, found that when depression and anxiety coexist, the symptoms are likely to be more severe and pose a higher risk of PTB.7 Similar to our findings, both maternal smoking and alcohol consumption can increase the risk of PTB.46-50 Unlike other studies,24,29 however, our study did not find that age played a significant role in predicting PTB among women with prepregnancy and prenatal depression and anxiety. This may be because only 13.4% of this sample fell in the high-risk categories of under 18 or over 35 years of age. Liu, et al, reported that prepregnancy obesity is associated with PTB in the general population, which is similar to this study’s findings of a mild association between obesity (BMI >29) and PTB.
The finding of Hispanic/Latina ethnicity as a protective factor against PTB is also in line with existing literature.26, 27, 46 Future research should delve further into reasons that Hispanic/Latina ethnicity may be protective, including through increased social support, as has been hypothesized.26, 27, 46
Health Implications
Pregnancy is a particularly vulnerable time for mothers, and the cumulative impact of prepregnancy and prenatal depression and anxiety can affect maternal mood and increase the likelihood of PTB and impaired fetal development. This, in turn, can lead to long-lasting psychological and neurological effects for the child.32 Additional research with a larger, more diverse group is needed to further test the overall associations between a mother’s age, race, ethnicity, and prepregnancy and prenatal depression and anxiety, and PTB.52,53 Mental health screening during prepregnancy, prenatal, and postpartum visits is imperative to develop appropriate mitigation measures.54
Conclusion
By looking at the existence of both depression and anxiety from prepregnancy to birth, the prevalence and cumulative impact of these mental disorders and their association with experiencing PTB can be better understood. This study highlights a positive association, which translates into a higher risk for PTB among women who experience the cumulative impact of prepregnancy and prenatal depression and anxiety, compared to those with only one disorder or none at all. The finding of an effect modification by Hispanic/Latina ethnicity emphasizes the impact of, most likely, social factors associated with maternal ethnicity and the risk of PTB.
Acknowledgements
Data were provided by the Utah Pregnancy Risk Assessment and Monitoring System (PRAMS), a project of the Utah Department of Health (UDOH), the Office of Vital Records and Health Statistics of the UDOH, and the Centers for Disease Control Prevention and Prevention (CDC) of the US Health and Human Services Department. This report does not represent the official views of the CDC or UDOH. We thank Dr. Charles Rogers for his insightful comments and critiques of various versions of the manuscript.
Funding: Research was partially supported by National Institute of Aging (NIA) grants “Hypertensive Disorders of Pregnancy and Subsequent Risk of Vascular Dementia, Alzheimer’s Disease, or Related Dementia: A Retrospective Cohort Study Taking into Account Mid-Life Mediating Factors” (Project K01AG058781; PI: Karen Schliep)
References
- A Pregnancy Risk Assessment Monitoring System Report – January 2021 Maternal Mental Health in Utah. PRAMS perspectives. https://mihp.utah.gov/wp-content/uploads/Maternal-Mental-Health-Utah-PRAMS-2016-2019.pdf. Published 2021. Accessed May 8, 2022.
- Effects of Maternal Age and Age-Specific Preterm Birth Rates on Overall Preterm Birth Rates — United States, 2007 and 2014. https://www.cdc.gov/mmwr/volumes/65/wr/mm6543a1.htm. Accessed May 8, 2022.
- Preterm Birth. https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Published 2021. Accessed June 17, 2021.
- Biaggi A, Conroy S, Pawlby S, Pariante C. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord. 2016; 191:62-77. doi:10.1016/j.jad.2015.11.014
- Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25(2):141-148. doi:10.1097/yco.0b013e3283503680
- Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Practice Research Clinical Obstetrics Gynaecology. 2014;28(1):25-35. doi:10.1016/j.bpobgyn.2013.08.017
- Kinsella M, Monk C. Impact of Maternal Stress, Depression and Anxiety on Fetal Neurobehavioral Development. Clin Obstet Gynecol. 2009;52(3):425-440. doi:10.1097/grf.0b013e3181b52df1
- Healthy People 2010. 2nd ed. Washington, DC: U.S. Dept. of Health and Human Services; 2000.
- Major Depression. National Institute of Mental Health (NIMH). https://www.nimh.nih.gov/health/statistics/major-depression#part_155029. Published 2022. Accessed May 8, 2022.
- Adhikari K, Patten S, Williamson T et al. Neighbourhood socioeconomic status modifies the association between anxiety and depression during pregnancy and preterm birth: a Community-based Canadian cohort study. BMJ Open. 2020;10(2):e031035. doi:10.1136/bmjopen-2019-031035
- Marcus S, Flynn H, Blow F, Barry K. Depressive Symptoms among Pregnant Women Screened in Obstetrics Settings. J Womens Health. 2003;12(4):373-380. doi:10.1089/154099903765448880
- Fellenzer J, Cibula D. Intendedness of Pregnancy and Other Predictive Factors for Symptoms of Prenatal Depression in a Population-Based Study. Matern Child Health J. 2014;18(10):2426-2436. doi:10.1007/s10995-014-1481-4
- Edwards B, Galletly C, Semmler-Booth T, Dekker G. Antenatal Psychosocial Risk Factors and Depression Among Women Living in Socioeconomically Disadvantaged Suburbs in Adelaide, South Australia. Australian & New Zealand Journal of Psychiatry. 2008;42(1):45-50. doi:10.1080/00048670701732673
- Bayrampour H, McDonald S, Tough S. Risk factors of transient and persistent anxiety during pregnancy. Midwifery. 2015;31(6):582-589. doi:10.1016/j.midw.2015.02.009
- Giardinelli L, Innocenti A, Benni L et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2011;15(1):21-30. doi:10.1007/s00737-011-0249-8
- Martini J, Petzoldt J, Einsle F, Beesdo-Baum K, Höfler M, Wittchen H. Risk factors and course patterns of anxiety and depressive disorders during pregnancy and after delivery: A prospective-longitudinal study. J Affect Disord. 2015;175:385-395. doi:10.1016/j.jad.2015.01.012
- Nasreen H, Kabir Z, Forsell Y, Edhborg M. Prevalence and associated factors of depressive and anxiety symptoms during pregnancy: A population based study in rural Bangladesh. BMC Womens Health. 2011;11(1). doi:10.1186/1472-6874-11-22
- Lancaster C, Gold K, Flynn H, Yoo H, Marcus S, Davis M. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5-14. doi:10.1016/j.ajog.2009.09.007
- Verreault N, Da Costa D, Marchand A, Ireland K, Dritsa M, Khalifé S. Rates and risk factors associated with depressive symptoms during pregnancy and with postpartum onset. Journal of Psychosomatic Obstetrics & Gynecology. 2014;35(3):84-91. doi:10.3109/0167482x.2014.947953
- Goldenberg R, Culhane J, Iams J, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371(9606):75-84. doi:10.1016/s0140-6736(08)60074-4
- Kotelchuck M. Pregnancy Risk Assessment Monitoring System (PRAMS): Possible New Roles for a National MCH Data System. Public Health Rep. 2006;121(1):6-10. doi:10.1177/003335490612100105
- Shulman H, D’Angelo D, Harrison L, Smith R, Warner L. The Pregnancy Risk Assessment Monitoring System (PRAMS): Overview of Design and Methodology. Am J Public Health. 2018;108(10):1305-1313. doi:10.2105/ajph.2018.304563
- Pregnancy Risk Assessment Monitoring System (PRAMS). https://www.cdc.gov/prams/index.htm. Accessed May 8, 2022.
- Maternal and Infant Health Program: PRAMS. https://mihp.utah.gov/pregnancy-and-risk-assessment. Accessed May 8, 2022.
- Utah Quick Facts. https://www.census.gov/quickfacts/UT. Published 2020. Accessed May 8, 2022.
- Martin J. United States vital statistics and the measurement of gestational age. Paediatr Perinat Epidemiol. 2007;21(s2):13-21. doi:10.1111/j.1365-3016.2007.00857
- Committee Opinion No 700: Methods for Estimating the Due Date. Obstetrics & Gynecology. 2017;129(5):e150-e154. doi:10.1097/aog.0000000000002046
- Salomon L, Alfirevic Z, Da Silva Costa F et al. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound in Obstetrics & Gynecology. 2019;53(6):715-723. doi:10.1002/uog.20272
- Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS One. 2018;13(1):e0191002. doi:10.1371/journal.pone.0191002
- Szegda K, Markenson G, Bertone-Johnson E, Chasan-Taber L. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. The Journal of Maternal-Fetal & Neonatal Medicine. 2013;27(9):960-967. doi:10.3109/14767058.2013.845157
- Manuck T. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin Perinatol. 2017;41(8):511-518. doi:10.1053/j.semperi.2017.08.010
- Watson L, Rayner J, King J, Jolley D, Forster D, Lumley J. Modelling prior reproductive history to improve prediction of risk for very preterm birth. Paediatr Perinat Epidemiol. 2010;24(5):402-415. doi:10.1111/j.1365-3016.2010.01134.x
- Atkinson K, Nobles C, Kanner J, Männistö T, Mendola P. Does maternal race or ethnicity modify the association between maternal psychiatric disorders and preterm birth?. Ann Epidemiol. 2021;56:34-39.e2. doi:10.1016/j.annepidem.2020.10.009
- Santos C, Fiaccone R, Oliveira N et al. Estimating adjusted prevalence ratio in clustered cross-sectional epidemiological data. BMC Med Res Methodol. 2008;8:80. doi:10.1186/1471-2288-8-80
- Petersen M, Deddens J. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008;8:9. doi:10.1186/1471-2288-8-9
- Mason S, Kaufman J, Daniels J, Emch M, Hogan V, Savitz D. Neighborhood ethnic density and preterm birth across seven ethnic groups in New York City. Health Place. 2011;17(1):280-288. doi:10.1016/j.healthplace.2010.11.006
- A Pregnancy Risk Assessment Monitoring System Report – January 2021 Maternal Mental Health in Utah. PRAMS perspectives. https://mihp.utah.gov/wp-content/uploads/Maternal-Mental-Health-Utah-PRAMS-2016-2019.pdf. Published 2021. Accessed May 8, 2022.
- Accortt E, Cheadle A, Dunkel Schetter C. Prenatal Depression and Adverse Birth Outcomes: An Updated Systematic Review. Matern Child Health J. 2014;19(6):1306-1337. doi:10.1007/s10995-014-1637-2
- What are possible causes of stillbirth?. National Institute of Health. https://www.nichd.nih.gov/health/topics/stillbirth/topicinfo/causes. Accessed May 8, 2022.
- Le H. Linking MCH and WIC: Integrating perinatal depression screening and prevention for high risk pregnant women | MCHB. HRSA. https://mchb.hrsa.gov/research/project_info.asp?ID=143. Published 2022. Accessed May 9, 2022.
- A Pregnancy Risk Assessment Monitoring System Report – January 2021 Maternal Mental Health in Utah. PRAMS perspectives. https://mihp.utah.gov/wp-content/uploads/Maternal-Mental-Health-Utah-PRAMS-2016-2019.pdf. Published 2021. Accessed May 8, 2022.
- Norhayati M, Nik Hazlina N, Asrenee A, Wan Emilin W. Magnitude and risk factors for postpartum symptoms: A literature review. J Affect Disord. 2015;175:34-52. doi:10.1016/j.jad.2014.12.041
- Should Race Be Used as a Variable in Research on Preterm Birth?. AMA J Ethics. 2018;20(3):296-302. doi:10.1001/journalofethics.2018.20.3.sect1-1803
- DeFranco E, Hall E, Muglia L. Racial disparity in previable birth. Am J Obstet Gynecol. 2016;214(3):394.e1-394.e7. doi:10.1016/j.ajog.2015.12.034
- Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women and Birth. 2015;28(3):179-193. doi:10.1016/j.wombi.2015.02.003
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6:125-140. doi:10.1080/14622200410001669187
- Ikehara S, Kimura T, Kakigano A et al. Association between maternal alcohol consumption during pregnancy and risk of preterm delivery: the Japan Environment and Children’s Study. BJOG: An International Journal of Obstetrics & Gynaecology. 2019;126(12):1448-1454. doi:10.1111/1471-0528.15899
- Hamułka J, Zielińska MA, Chądzyńska K. The combined effects of alcohol and tobacco use during pregnancy on birth outcomes. Rocz Panstw Zakl Hig. 2018;69(1):45-54.
- Abuidhail J, Abujilban S. Characteristics of Jordanian depressed pregnant women: a comparison study. J Psychiatr Ment Health Nurs. 2013;21(7):573-579. doi:10.1111/jpm.12125
- Räisänen S, Lehto S, Nielsen H, Gissler M, Kramer M, Heinonen S. Risk factors for and perinatal outcomes of major depression during pregnancy: a population-based analysis during 2002–2010 in Finland. BMJ Open. 2014;4(11):e004883. doi:10.1136/bmjopen-2014-004883
- Mercer B, Goldenberg R, Moawad A et al. The Preterm Prediction Study: Effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol. 1999;181(5):1216-1221. doi:10.1016/s0002-9378(99)70111-0
- Liu B, Xu G, Sun Y et al. Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: a population-based study. The Lancet Diabetes & Endocrinology. 2019;7(9):707-714. doi:10.1016/s2213-8587(19)30193-7
- Lynch A, Hart J, Agwu O, Fisher B, West N, Gibbs R. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am J Obstet Gynecol. 2014;210(5):428.e1-428.e9. doi:10.1016/j.ajog.2013.12.011
- Bauman B, Ko J, Cox S et al. Vital Signs: Postpartum Depressive Symptoms and Provider Discussions About Perinatal Depression — United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):575-581. doi:10.15585/mmwr.mm6919a2
Citation
Seage M, Petersen M, Carlson M, VanDerslice J, Stanford J, & Schliep K. (2022). What Role Does Hispanic/Latina Ethnicity Play in the Relationship Between Maternal Mental Health and Preterm Birth? Utah Women’s Health Review. doi: 10.26054/0d-dkas-c5qe

