Table of Contents
Abstract
Objectives: The objective of this study is to test the association between preconception polycystic ovary syndrome (PCOS) and gestational diabetes mellitus (GDM) using Utah’s Pregnancy Risk Assessment Monitoring System (2016-2021). In addition, pre-pregnancy hypertension will be tested as a potential effect moderator.
Methods: This cross-sectional study utilizes data from Phase 8 of the Utah Pregnancy Risk Assessment Monitoring System (PRAMS) survey (2016-2021). The association between PCOS and GDM was tested using Poisson regression to generate adjusted prevalence ratios and 95% confidence intervals.
Results: PCOS was associated with higher prevalence of GDM in all models, regardless of whether the outcome data (GDM) came from the infant’s birth certificate, the PRAMS survey, or the combined measure. When adjusting for sociodemographic characteristics, lifestyle factors, reproductive history, and comorbidities, women with PCOS were 1.50 (1.16-1.95) times as likely to have GDM (reported on birth certificate and/or survey) compared to women without PCOS. Pre-pregnancy hypertension was not found to be a statistically significant effect moderator.
Conclusion: The findings from this study were consistent with the majority of research indicating that women with PCOS have increased risk for GDM. This is also the first known study to test pre-pregnancy hypertension as an effect moderator between PCOS and GDM. More research is needed on the role of comorbidities such as chronic hypertension as effect modifiers between PCOS and GDM.
Implications: These findings show that women with PCOS are at high risk for GDM, among a population-based sample of mothers. Interventions to reduce the risk of GDM among women with PCOS need to be developed and evaluated.
Introduction
An estimated 5-15% of women have polycystic ovary syndrome (PCOS).1 The three diagnostic features according to the Rotterdam Criteria are ovulatory dysfunction such as anovulation and oligo-ovulation, hyperandrogenism, and polycystic ovaries. Patients must display two of the three to be diagnosed.2 In addition, clinicians must exclude other conditions with similar presentations, such as congenital adrenal hyperplasia, Cushing’s syndrome and androgen-secreting tumors, and drug-induced androgen excess.2
PCOS is a major contributor to infertility. Ovulatory disorders account for approximately 25% of infertility diagnoses; 70% of women with anovulation have polycystic ovary syndrome.3 In addition to being at increased risk for infertility, women with PCOS have elevated risk of obesity, type II diabetes, hyperlipidemia, depression, anxiety, obstructive sleep apnea, nonalcoholic fatty liver disease, endometrial cancer, and cardiovascular disease.4,5 The cause of PCOS is unknown and there is no cure.
Gestational diabetes mellitus (GDM) is a glucose tolerance disorder that starts during pregnancy and affects approximately 6% of pregnancies. The prevalence of GDM has had a relative increase of approximately 78% in the last two decades. The rising prevalence of GDM over the last few decades is concerning because GDM is associated with pre-eclampsia, macrosomia, and future metabolic diseases in the mother and child.6
Prior studies have produced mixed findings regarding the presence and magnitude of the association between PCOS and GDM. The majority of the literature including the most recent meta-analysis found that women with PCOS have elevated risk of GDM compared to women without PCOS.6 The objective of this study is to test the association between preconception PCOS and GDM using Utah’s Pregnancy Risk Assessment Monitoring System (2016-2021). Utilizing these data allows the association between preconception PCOS and GDM to be tested among a representative population-based sample. This study includes women who are often underrepresented in hospital or clinic-based studies, such as Hispanics and women of low socioeconomic status.
Another unique feature of this study is that pre-pregnancy hypertension will be tested as a potential effect moderator between PCOS and GDM. PCOS is considered a heterogeneous disorder with multiple phenotypes which affect a person’s health risks at different stages of life.7 The purpose of testing pre-pregnancy hypertension as an effect moderator is to determine if the risk of GDM differs among women with and without pre-pregnancy hypertension. This information could contribute to our understanding of the underlying mechanism between PCOS and GDM as well as inform clinical decisions and treatment.7
Methods
Study Design
This cross-sectional study utilizes data from the 2016-2021 Utah Pregnancy Risk Assessment Monitoring System (PRAMS) survey which is a joint project between state, local, tribal, and territorial health departments and the Centers for Disease Control and Prevention (CDC).
Data Sources
PRAMS data collection began in 1987 to understand why some infants are born healthy and others are not. It was designed to identify groups of women and infants at high risk for health problems, monitor changes in health status, and measure progress toward goals in improving the health of mothers and infants.8 PRAMS surveillance began in Utah in 1999.9
Approximately 200 new mothers are randomly selected to participate each month from Utah birth certificates.9 Recruitment for PRAMS occurs according to the protocol developed by the CDC which combines statewide mailings of the surveys and telephone follow-up to women who do not complete the survey by mail. A key feature of PRAMS is the stratified systematic sampling, which over samples from populations of interest based on factors such as maternal age, race/ethnicity, geographic area of residence, and infant birth weight.10 The survey is available in both English and Spanish.
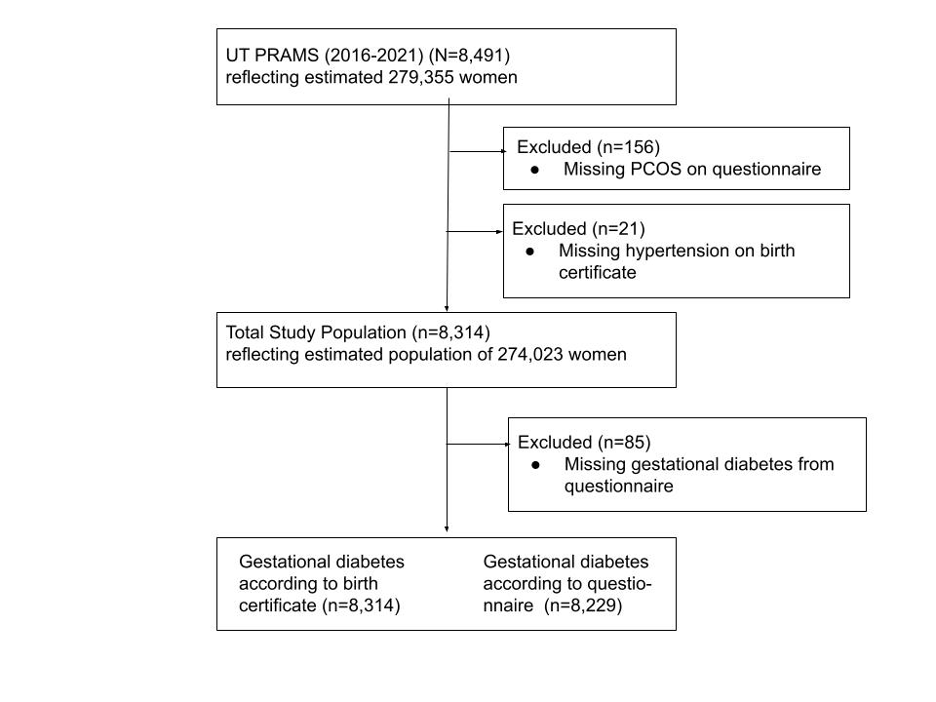
The data used in this study were from the Utah PRAMS Phase 8 (2016-2021) questionnaire (n=8,491) reflecting an estimated population of 279,355 women. The design and sampling frame assure the study sample is representative of Utah’s population. The unweighted response rates in Utah were 55.6% in 2016, 60.2% in 2017, 54% in 2018, 69.5% in 2019, 60.9% in 2020, and 52.6% in 2021.8
Outcome Measures
The outcome of interest for this study was whether or not a woman was diagnosed with gestational diabetes mellitus (GDM) during her most recent pregnancy. This outcome was measured in two ways. First, as a field on the birth certificate and second as a self-reported item on the PRAMS survey. On the survey women were asked, “during your most recent pregnancy, did you have any of the following health conditions?” One of the listed conditions was, “gestational diabetes (diabetes that started during this pregnancy)” and respondents were instructed to check yes or no. Estimates of the association between preconception polycystic ovary syndrome (PCOS) and gestational diabetes mellitus (GDM) will be provided using each outcome measurement method separately as well as a combined measure, which includes mothers who self-reported GDM for index pregnancy via PRAMS survey and those for whom GDM was indicated on the birth certificate. 8012 observations (96.4%) were categorized consistently for GDM between the survey and the birth certificate. However, 85 respondents did not self-report whether or not they had GDM on the survey and 59 respondents were categorized as not having GDM on the survey though it was indicated on the birth certificate. Conversely, 158 respondents self-reported having GDM on the survey though it was not indicated on the birth certificate. The combined GDM measure is the most inclusive outcome measurement and classifies observations as having had GDM if they self-reported GDM on the survey or if GDM was indicated on the birth certificate.
In prior studies, both birth certificate data and maternal recall have demonstrated excellent validity for variables related to labor and delivery when compared to medical records.11 Furthermore, one study using New York state PRAMS data found good agreement between birth certificate and self-reporting on the PRAMS survey specifically for GDM.12 Though both measurement methods are believed to have a high degree of validity, due to the 85 missing values for GDM on the survey, the birth certificate record may be the most valid measurement method for this study.
Covariates
Potential confounding factors believed to influence both PCOS and GDM were determined based on prior literature.1,4,5,6 Sociodemographic covariates include maternal age in years, yearly total household income before taxes during the 12 months before the new baby was born (categorical), maternal education (categorical), race/ethnicity (white/non-white and Hispanic/non-Hispanic), marital status (married/not married), and urban/rural residence. Salt Lake, Davis, Utah, and Weber counties were categorized as urban, and all others were categorized as rural. Lifestyle factors included cigarette smoking within the last two years (yes/no), drinking within the last two years (yes/no), and pre-pregnancy BMI (categorical). Reproductive history and comorbidities including previous live birth (yes/no), infertility treatment (yes/no), depression during the three months before getting pregnant (yes/no), and anxiety during the three months before getting pregnant (yes/no) were also considered as potential confounding factors. The following covariates were collected on the survey: maternal age, family income, cigarette smoking, drinking, pre-pregnancy BMI, pre-pregnancy depression, and pre-pregnancy anxiety. The following covariates were collected on the birth certificate: maternal education, race/ethnicity, marital status, urban/rural residence, previous live birth, and infertility treatment.
Respondents self-reported hypertension during the three months before getting pregnant (yes/no) on the survey. Pre-pregnancy hypertension was tested as an effect moderator is to determine if the risk of GDM differs among women with PCOS with and without comorbid hypertension.
Statistical Analysis
After excluding observations with missing values for PCOS (n=156) and hypertension (n=21), 8314 women (97.9%) were included in the analyses, reflecting an estimated population size of 274,023 women (Figure 1). Participant characteristics were reported by PCOS status, the exposure of interest. All variables were categorical and are reported as weighted percentages. Descriptive characteristics of respondents with and without PCOS were calculated by chi-square tests, taking into account the stratified survey sampling.
The association between PCOS and gestational diabetes was tested using Poisson regression models with a robust error variance. The models accounted for PRAMS’ use of stratified sampling and generated adjusted prevalence ratios (PR) and 95% confidence intervals (CI). The reference group in all models was mothers without PCOS. Pre-pregnancy hypertension was tested as an effect modifier using a stratified analysis and the Wald test. SAS Studio 9.4 and Stata 16.1 were used for data analysis.
Results
Characteristics of the Mothers
The majority of the respondents were non-Hispanic white (77.7%), received education beyond high school (70.4%), were married (81.7%), and lived in an urban area (76.4%) (Table 1). The median maternal age was 28.5 among respondents with PCOS and 27.9 among respondents without PCOS (Table 1). Most of the women were parous (65.4%), had a normal pre-pregnancy BMI (47.9%), and had not smoked cigarettes (90.7%) or drank alcohol (65.6%) within the last two years before becoming pregnant (Table 1).
Table 1. Sociodemographic, lifestyle and clinical characteristics of women by polycystic ovary syndrome (PCOS) status, Utah PRAMS, 2016-2021, n=8,314, reflecting an estimated population size of 274,023 women
| Characteristic | Overall | No PCOS | PCOS | P-value (chi-square) |
| Maternal Age (%) | ||||
| 15-19 | 3.2 | 3.3 | 1.4 | |
| 20-29 | 54.3 | 54.4 | 52.7 | |
| 30-39 | 40.0 | 39.7 | 43.0 | |
| 40+ | 2.6 | 2.6 | 3.0 | 0.08 |
| Family Income (%) | ||||
| $0-$20,000 | 14.2 | 14.5 | 11.3 | |
| $20,001-$32,000 | 13.5 | 13.7 | 10.7 | |
| $32,001-$57,000 | 22.1 | 22.1 | 22.3 | |
| $57,001-$85,000 | 20.9 | 20.8 | 22.3 | |
| $85,001+ | 23.6 | 23.1 | 29.4 | <0.00 |
| Maternal Education (%) | ||||
| Less than HS | 7.4 | 7.7 | 4.3 | |
| HS/GED | 19.6 | 19.7 | 17.4 | |
| Some College | 21.8 | 21.8 | 22.0 | |
| Associate | 10.7 | 10.5 | 13.1 | |
| Bachelors | 30.1 | 29.9 | 32.1 | |
| Masters, Doctorate/Professional | 7.8 | 7.7 | 8.6 | 0.09 |
| Race/ethnicity (%) | ||||
| Hispanic, non-white | 4.2 | 4.4 | 2.0 | |
| Hispanic, white | 11.9 | 12.0 | 11.1 | |
| Non-Hispanic, white | 77.7 | 77.3 | 82.5 | |
| Non-Hispanic, non-white | 6.0 | 6.2 | 4.1 | 0.01 |
| Married (%) | 81.7 | 81.5 | 84.5 | 0.13 |
| Rural (%) | 23.6 | 23.7 | 21.4 | 0.30 |
| Smoking (%) | 9.3 | 9.4 | 9.3 | 0.07 |
| Drinking (%) | 34.4 | 34.2 | 36.4 | 0.52 |
| Body Mass Index (BMI) (%) | ||||
| Underweight (<18.5) | 3.8 | 4.0 | 1.7 | |
| Normal (18.5-24.9) | 47.9 | 49.2 | 31.3 | |
| Overweight (25.0-29.9) | 24.2 | 24.3 | 22.7 | |
| Obese (30.0+) | 22.9 | 21.2 | 42.8 | |
| Unknown | 1.3 | 1.3 | 1.5 | <0.00 |
| Depression (%) | 18.5 | 17.1 | 35.9 | <0.00 |
| Anxiety (%) | 28.1 | 26.4 | 48.8 | <0.00 |
| Hypertension (%) | 3.9 | 2.4 | 22.5 | <0.00 |
| Previous live birth (%) | 65.4 | 65.7 | 62.6 | 0.21 |
| Infertility treatment (%) | 6.8 | 5.0 | 30.1 | <0.00 |
b. Cigarette smoking and drinking up to two years before pregnancy
c. Depression, anxiety, and hypertension diagnoses prior to pregnancy
2. Missing frequencies for characteristics: family income n=552 (6.6%), maternal education n=321 (3.9%), race/ethnicity n=17 (0.2%), smoking n=84 (1.0%), drinking n=112 (1.3%), depression n=15 (0.2%), anxiety n=16 (0.2%)
Compared to women without PCOS, women with PCOS were more likely to be non-Hispanic white, have higher family income, be older, and have higher educational attainment (Table 1). Women with PCOS also had significantly higher rates of obesity (42.8% vs. 21.2%), depression (35.9% vs. 17.1%), anxiety (48.8% vs. 26.4%), hypertension (22.5% vs. 2.4%), and utilization of infertility treatment (30.1% vs. 5.0%) compared to women without PCOS (Table 1).
Association Between Polycystic Ovary Syndrome (PCOS) and Gestational Diabetes Mellitus (GDM)
PCOS was associated with higher prevalence of GDM in all models, regardless of whether the outcome data came from the infant’s birth certificate, the PRAMS survey, or the combined measure. Estimates did not meaningfully differ between the different GDM variables (Table 2). After adjusting for sociodemographic characteristics of age, educational attainment, family income, race/ethnicity, marital status, and urban/rural, women with PCOS were 2.14 times (95% CI: 1.63–2.81) as likely to have GDM using the birth certificate measure, 2.0 times (95% CI: 1.54–2.60) as likely to have GDM using the PRAMS survey measure, and 2.02 times (95% CI: 1.57–2.59) as likely to have GDM using the combined measure compared to women without PCOS (Table 2).
After further adjustment by lifestyle factors of cigarette smoking, alcohol drinking, and BMI, women with PCOS were 1.64 times (95% CI: 1.24–2.17) as likely to have GDM using the birth certificate measure, 1.56 times (95% CI: 1.20–2.04) as likely to have GDM using the PRAMS survey measure, and 1.58 times (95% CI: 1.23–2.03) as likely to have GDM using the combined measure compared to women without PCOS (Table 2).
The final model was further adjusted by reproductive history and comorbidities including previous live birth, infertility treatment, depression, anxiety, and hypertension. In the final model women with PCOS were 1.49 (95% CI: 1.11–2.01) times as likely to have GDM using the birth certificate measure, 1.53 times (95% CI: 1.16–2.01) times as likely to have GDM using the PRAMS survey measure, and 1.50 (95% CI 1.16–1.95) times as likely to have GDM using the combined measure compared to women without PCOS (Table 2).
Table 2. Association between polycystic ovary syndrome (PCOS) and gestational diabetes mellitus (GDM)
| Gestational Diabetes (GDM) | |||||
| Unadjusted PR (95% CI) | Model 1 PR (95% CI) | Model 2 PR (95% CI) | Model 3 PR (95% CI) | ||
| GDM – Birth Certificate | |||||
| PCOS | 2.04 (1.56-2.68) | 2.14 (1.63-2.81) | 1.64 (1.24-2.17) | 1.49 (1.11-2.01) | |
| No PCOS | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
| GDM – Survey | |||||
| PCOS | 1.94 (1.51-2.51) | 2.00 (1.54-2.60) | 1.56 (1.20-2.04) | 1.53 (1.16-2.01) | |
| No PCOS | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
| GDM – Birth Certificate or Survey | |||||
| PCOS | 1.92 (1.50-2.45) | 2.02 (1.57-2.59) | 1.58 (1.23-2.03) | 1.50 (1.16-1.95) | |
| No PCOS | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
b. Model 1 was adjusted by sociodemographic characteristics of maternal age, maternal education, family income, race/ethnicity, marital status, and urban/rural
c. Model 2 was further adjusted by lifestyle factors of cigarette smoking, drinking, and BMI
d. Model 3 was further adjusted by reproductive history or comorbidities of previous live birth, infertility treatment, depression, anxiety, and hypertension
e. Poisson regression models with robust error variance, taking into account stratified sampling used to calculate PRs and 95% CIs
Association Between Polycystic Ovary Syndrome (PCOS) and Gestational Diabetes Mellitus (GDM) Stratified by Pre-Pregnancy Hypertension Status
Unexpectedly, the results of the stratified analysis suggest that women with PCOS who did not have comorbid pre-pregnancy hypertension had higher prevalence of GDM than women with PCOS and comorbid pre-pregnancy hypertension. However, the results were not statistically significant using the Wald test in models that adjusted for lifestyle factors, reproductive history, and comorbidities. Women with PCOS who did not have comorbid pre-pregnancy hypertension did have significantly higher risk of GDM in all models compared to women without PCOS (Table 3).
Table 3. Relationship between polycystic ovary syndrome and gestational diabetes mellitus, stratified by hypertension status
| Gestational Diabetes (GDM) | |||||
| Unadjusted PR (95% CI) | Model 1 | Model 2 | Model 3 | ||
| GDM – Birth Certificate | |||||
| PCOS and Hypertension | 1.27 (0.67-2.38) | 1.20 (0.62-2.33) | 1.01 (0.52-1.98) | 1.08 (0.56-2.11) | |
| PCOS without Hypertension | 2.27 (1.70-3.03) | 2.41 (1.81-3.22) | 1.80 (1.34-2.42) | 1.60 (1.16-2.21) | |
| Wald Test for Hypertension | 0.02 | 0.04 | 0.27 | 0.41 | |
| GDM – Survey | |||||
| PCOS and Hypertension | 1.37 (0.79-2.36) | 1.13 (0.61-2.10) | 0.96 (0.52-1.81) | 1.09 (0.59-2.04) | |
| PCOS without Hypertension | 2.11 (1.60-2.78) | 2.25 (1.71-2.97) | 1.72 (1.30-2.28) | 1.64 (1.21-2.22) | |
| Wald Test for Hypertension | 0.01 | 0.01 | 0.08 | 0.14 | |
| GDM – Birth Certificate or Survey | |||||
| PCOS and Hypertension | 1.31 (0.78-2.21) | 1.13 (0.63-2.03) | 0.97 (0.53-1.76) | 1.08 (0.59-1.97) | |
| PCOS without Hypertension | 2.10 (1.61-2.74) | 2.27 (1.74-2.96) | 1.74 (1.33-2.26) | 1.61 (1.22-2.14) | |
| Wald Test for Hypertension | 0.00 | 0.00 | 0.05 | 0.11 | |
b. The reference group was women without PCOS for each estimate
c. Model 1 was adjusted by sociodemographic characteristics of maternal age, maternal education, family income, race/ethnicity, marital status, and urban/rural
d. Model 2 was further adjusted by lifestyle factors of cigarette smoking, drinking, and BMI
e. Model 3 was further adjusted by reproductive history or comorbidities of previous live birth, infertility treatment, depression, anxiety, and hypertension
f. Poisson regression models with robust error variance, taking into account stratified sampling used to calculate PRs and 95% CIs
Discussion
The primary finding of this statewide sample of mothers 2-4 months postpartum indicate that women who reported having been diagnosed with preconception PCOS had significantly higher prevalence of GDM compared to women without PCOS when controlling for confounding variables. Hypertension was not found to be a statistically significant effect moderator. This test was done to contribute to our understanding of the underlying mechanism between PCOS and GDM as well as inform clinical care. However, it’s possible the effect moderator was not significant because of the small number of women with both PCOS and hypertension (n=158).
It is also possible that pregnant women with PCOS who do not have comorbid pre-pregnancy hypertension do not receive the same extent of clinical care as women with comorbid conditions and this contributes to their elevated risk of GDM. Women with chronic hypertension, high blood pressure at or above 140/90 mmHg before getting pregnant, are at higher risk of preeclampsia, which is a leading cause of maternal mortality.7,13 This elevated risk may cause women with pre-pregnancy hypertension to receive more care than pregnant women without hypertension. For example, women with pre-pregnancy hypertension are recommended to receive preconception health care, discuss medications that are safe to take during pregnancy, monitor blood pressure at home, and make lifestyle modifications to promote a healthy diet and regular physical activity.13 These interventions designed to reduce the risk of hypertensive disorders of pregnancy (HDP) may also reduce the risk of GDM.
Interpretation
The findings from this study were consistent with the majority of research including three previous meta-analyses. Yu et al. (2016) reported that women with PCOS showed an elevated prevalence of GDM (RR = 2.78, 95% CI: 2.27–3.40).6 Khomami et al. (2019) reported women with PCOS had a higher prevalence of GDM (OR = 2.89, 95% CI: 2.37–3.54)14 and Boomsma et al. (2006). found that PCOS in pregnancy significantly increased the risk of GDM (OR = 2.94, 95% CI: 1.70–5.08).15 Though the risk of GDM was elevated among women with pre-pregnancy PCOS, the estimates for this study using a population-based cohort of women in Utah were lower than the risk ratios and odds ratios of the meta-analyses by Yu et al. (2016), Khomami et al. (2019), and Boomsma et al. (2006). This could be due to Utah’s population being a younger and healthier population compared to the populations of the other studies, which were mainly comprised of women recruited from clinical settings.
Limitations
Limitations of this study include the retrospective nature of the PRAMs questionnaire, which limits the ability to infer causality. The questionnaire collects information from postpartum mothers about experiences they had before and during pregnancy which depend on participant’s accurate recall. Social desirability may also have impacted the collection of data on potential confounders since new mothers may not wish to report certain lifestyle behaviors. Another limitation is that that the PRAMS survey is restricted to women who successfully had a live birth. Women with PCOS may be underrepresented because of subfertility. This is important because the results may differ if women who have not yet conceived were included. Classifying women with PCOS based on self-reported diagnosis likely underestimates the number of women with PCOS, compared to using self-reported symptoms to identify women with PCOS. However, this misclassification of the exposure biases the results toward the null. Even more broadly, the lack of a biomarker that can be used in PCOS diagnosis is a significant limitation that affects all research on PCOS. Finally, this dataset did not have information on PCOS phenotype which would have been useful in the analysis.
Strengths
Strengths of this study include the population-based sample which is representative of Utah due to its systematic stratified sampling scheme. This increases the generalizability of the results. The PRAMS questionnaire and linked birth records allowed for detailed information about sociodemographic characteristics, lifestyle, reproductive history, and comorbidities to be used to assess potential confounding. Finally, GDM was measured through the birth certificate, PRAMS survey, and through a combined measure.
Health Implications
These findings indicate that women with PCOS are at elevated risk for GDM, among a population-based sample of mothers in Utah. This is the first known study to test the association between PCOS and GDM in Utah but is consistent with prior literature in other populations indicating women with PCOS are at elevated risk for GDM when controlling for confounding factors. This finding should inform the development of clinical care guidelines, health promotion programs, and policies in Utah and locales with comparable populations. Interventions to reduce the risk of GDM among women with PCOS need to be developed and evaluated.
This is also the first known study to test hypertension as an effect moderator between PCOS and GDM. The purpose of testing hypertension as an effect moderator was to determine if the risk of GDM differs among women with PCOS who do and do not have pre-pregnancy hypertension. In this study, women with PCOS who did not have comorbid pre-pregnancy hypertension appeared to have higher prevalence of GDM than women with PCOS and comorbid hypertension. However, the results were not statistically significant in models that adjusted for lifestyle factors, reproductive history, and comorbidities. Future studies should explore whether in practice women with pre-pregnancy hypertension receive more clinical care than those without pre-pregnancy hypertension. If this is the case, and future research indicates that these interventions lower their risk of GDM, these interventions may also be beneficial to women with PCOS. More research is needed on the role of comorbidities such as hypertension as effect modifiers between PCOS and GDM, the causal mechanisms between PCOS and GDM, and interventions to prevent GDM among women with PCOS.
Acknowledgements
Data were provided by the Utah Pregnancy Risk Assessment Monitoring System, a project of the Utah Department of Health (UDOH), the Office of Vital Records and Health Statistics of the UDOH, and the Centers for Disease Control and Prevention (CDC) of the United States Department of Health and Human Services. The authors extend appreciation to the participants who made the study possible. This report does not represent the official views of the UDOH or CDC.
Funding
Dr. Karen Schliep was supported by a NIH National Institute of Aging (NIA) grant, “Hypertensive Disorders of Pregnancy and Subsequent Risk of Vascular Dementia, Alzheimer’s Disease, or Related Dementia: A Retrospective Cohort Study Taking into Account Mid-Life Mediating Factors” (K01AG058781).
References
- Azziz, Ricardo. “Introduction: Determinants of Polycystic Ovary Syndrome.” Fertility and Sterility, vol. 106, no. 1, 2016, pp. 4–5, https://doi.org/10.1016/j.fertnstert.2016.05.009.
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. “Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS).” Human Reproduction, vol. 19, no. 1, Jan. 2004, pp. 41–47, https://doi.org/10.1093/humrep/deh098.
- Carson, Sandra Ann, and Amanda N. Kallen. “Diagnosis and Management of Infertility: A Review.” JAMA, vol. 326, no. 1, July 2021, p. 65, https://doi.org/10.1001/jama.2021.4788.
- Shrivastava, Sneha, and Rosemarie L. Conigliaro. “Polycystic Ovarian Syndrome.” Medical Clinics of North America, vol. 107, no. 2, 2023, pp. 227–34, https://doi.org/10.1016/j.mcna.2022.10.004.
- Cooney, Laura G., and Anuja Dokras. “Beyond Fertility: Polycystic Ovary Syndrome and Long-Term Health.” Fertility and Sterility, vol. 110, no. 5, 2018, pp. 794–809, https://doi.org/10.1016/j.fertnstert.2018.08.021.
- Yu, Hai-Feng, et al. “Association between Polycystic Ovary Syndrome and the Risk of Pregnancy Complications: A PRISMA-Compliant Systematic Review and Meta-Analysis.” Medicine, vol. 95, no. 51, 2016, p. e4863, https://doi.org/10.1097/MD.0000000000004863.
- Mirza, Fadi G., et al. “Polycystic Ovarian Syndrome (PCOS): Does the Challenge End at Conception?” International Journal of Environmental Research and Public Health, vol. 19, no. 22, Nov. 2022, p. 14914, https://doi.org/10.3390/ijerph192214914.
- Centers for Disease Control and Prevention. PRAMS Methodology. 28 Mar. 2023, https://www.cdc.gov/prams/methodology.htm.
- Utah Department of Health & Human Services. “Maternal and Infant Health Program.” Utah PRAMS, https://mihp.utah.gov/pregnancy-and-risk-assessment. Accessed 18 July 2023.
- Shulman, Holly B., et al. “The Pregnancy Risk Assessment Monitoring System (PRAMS): Overview of Design and Methodology.” American Journal of Public Health, vol. 108, no. 10, 2018, pp. 1305–13, https://doi.org/10.2105/AJPH.2018.304563.
- Ziogas, Christina, et al. “Validation of Birth Certificate and Maternal Recall of Events in Labor and Delivery with Medical Records in the Iowa Health in Pregnancy Study.” BMC Pregnancy and Childbirth, vol. 22, no. 1, 2022, p. 232, https://doi.org/10.1186/s12884-022-04581-7.
- Hosler, Akiko S., et al. “Agreement Between Self-Report and Birth Certificate for Gestational Diabetes Mellitus: New York State PRAMS.” Maternal and Child Health Journal, vol. 14, no. 5, 2010, pp. 786–89, https://doi.org/10.1007/s10995-009-0529-3.
- CDC. “High Blood Pressure During Pregnancy.” Centers for Disease Control and Prevention, 19 June 2023, https://www.cdc.gov/bloodpressure/pregnancy.htm.
- Bahri Khomami, Mahnaz, et al. “Increased Maternal Pregnancy Complications in Polycystic Ovary Syndrome Appear to Be Independent of Obesity—A Systematic Review, Meta‐analysis, and Meta‐regression.” Obesity Reviews, vol. 20, no. 5, 2019, pp. 659–74, https://doi.org/10.1111/obr.12829.
- Boomsma, C. M., et al. “A Meta-Analysis of Pregnancy Outcomes in Women with Polycystic Ovary Syndrome.” Human Reproduction Update, vol. 12, no. 6, Aug. 2006, pp. 673–83, https://doi.org/10.1093/humupd/dml036.
Citation
Myrer RS, Adediran E, Ellsworth AD, Ceballos RM, Lopez I, Stanford JB, Talboys S, Wang J, Schliep KC. (2024). The association between preconception polycystic ovary syndrome and gestational diabetes mellitus among women with and without pre-pregnancy hypertension: a cross-sectional study from Utah’s Pregnancy Risk Assessment Monitoring System Survey (2016-2021). Utah Women’s Health Review. doi: 10.26054/d-k952-0keb

