Table of Contents
Abstract
Objectives: To describe differences in COVID-19 illness severity by race, ethnicity, age, and gender in a Utah population-based sample by examining the likelihood of severe outcomes along an expanded illness severity index.
Methods: This cross-sectional study uses clinical records of individuals tested for SARS-CoV-2 between March 10, 2020, and July 10, 2020, at University of Utah Health facilities. We utilized descriptive statistics and adjusted multinomial logistic regression to assess associations between demographic characteristics and COVID-19 illness severity.
Results: Of the 6445 individuals eligible for this study, 53.3 percent identified as female; 12.6 percent were ≥ 65 years of age; and 61.0 percent identified as White, non-Hispanic or Latino (WNH). Compared to WNH, racial and ethnic minoritized groups had a higher adjusted odds ratio (aOR) of receiving emergency department care or hospitalization (aOR =1.83 [95% CI: 1.09, 3.07]; aOR = 7.11 [95% CI: 2.90, 17.43], respectively). Compared to patients younger than 65 years, patients 65 years or older had a higher probability of receiving emergency department care or hospitalization (aOR =2.94 [95% CI: 1.46, 5.96]; aOR = 10.24 [95% CI: 5.53, 18.97], respectively). Male individuals, as compared to female, had similar probability of receiving emergency department care or hospitalization (OR = 0.72 [95% CI: 0.52, 1.0] and OR = 1.06 [95% CI: 0.80, 1.46], respectively).
Conclusions: We found that COVID-19 illness severity varied by race, ethnicity, and age characteristics in Utah, with greater illness severity disproportionately impacting racial and ethnic minoritized groups and those of greater age. We did not detect differences by gender in this sample.
Introduction
In mid-December 2019, a novel Coronavirus–SARS-CoV-2–was identified in a cluster of patients in Wuhan, Hubei Province, China.1,2 This virus quickly spread internationally, prompting the World Health Organization (WHO) to declare Novel Coronavirus Disease (COVID-19) a global pandemic on March 11, 2020.3 Now, more than 3 years after its initial detection and identification, over 100 million cases of COVID-19 have been identified in the United States, resulting in over 1 million deaths.4
While disease transmission and mortality are mitigated through vaccination and standard non-pharmaceutical interventions (i.e., physical distancing, masking, etc.), inequitable access to interventions, testing, and treatments combined with existing health disparities have allowed the burden of COVID-19 to persist. While health inequities vary geographically, racial and ethnic minoritized groups, including Black or African-Americans and Hispanic or Latino populations, bear a disproportionate burden of COVID-19-related health disparities, such as infection, morbidity, and mortality. Compared to White, non-Hispanic or Latino (WNH) individuals, racial and ethnic minoritized groups are more likely to contract COVID-19 due to living in higher-density residences and working in occupations requiring in-person presence.9, 11-14. These underserved communities also have reduced access to COVID-19 testing services.10,15
Racial and ethnic minoritized individuals have been identified as having more severe COVID-19 outcomes, such as hospitalization and death, compared to WNH.5-7, 7-19 While limited access to healthcare overall can partially explain race and ethnic disparities in COVID-19 morbidity and mortality,9,16 more detailed insights into COVID-19 outcome disparities are limited. Most studies focus on three levels of illness severity: testing positive, hospitalization, and mortality. The limited literature does not adequately address the severity variation experienced in non-hospitalized individuals.16,18,20
Given that racial and ethnic minoritized groups constitute over a quarter of the population in the United States,21 an understanding of how such populations are affected by COVID-19 illness severity is of great public health importance. Without knowledge of race and ethnicity’s effects on the risk of severe illness, targeted surveillance and preventive interventions for at-risk populations are hampered. Understanding the relationship between race, ethnicity, and COVID-19 severity will reduce modeling errors for COVID-19, increase targeted and effective allocation of public health assistance and outreach, and reduce COVID-19-related morbidity and mortality.6
To address prior knowledge gaps, this study aims to describe differences in COVID-19 outcome severity by race, ethnicity, and related sociodemographic factors in a Utah population-based sample with minimal selection bias.
Methods
Study Design and Sample Population
We conducted this retrospective cross-sectional study using clinical data of individuals tested for SARS-CoV-2 infections by the University of Utah’s academic healthcare system from March 10, 2020, to July 10, 2020. University of Utah Health, based in Salt Lake City, Utah, is an extensive healthcare network that oversees five regional hospitals and over 100 health centers and institutes, and 26 of these facilities interacted with COVID-19-positive individuals during the testing period.22 As a major referral center for the Intermountain West, University of Utah Health’s patient population comprises Utah and non-Utah residents. University of Utah IRB approval was obtained prior to the start of this study. We extracted clinical and demographic data from the University of Utah Electronic Data Warehouse. The clinical data used in this study included SARS-CoV-2 testing unit location and results; indications of select comorbidities; an indication of mechanical ventilation; an indication of in-hospital death (measured up to July 10, 2020); a general patient classification (outpatient, inpatient, emergency, institutional series, etc.); and admission and discharge date and time. Additionally, demographic data included self-reported race, ethnicity, gender identity, and age.
COVID-19 Predictors
The primary predictors of COVID-19 severity explored in this study were race, ethnicity, gender identity, and age. We explored multiple race and ethnicity combinations using self-reported race and ethnicity responses. The most granular race and ethnicity combination had 8 distinct responses. We created a collapsed race and ethnicity combination using this granular combination for statistical modeling and to protect patient confidentiality, as some demographic categories had limited observations (Table 1).

COVID-19 Outcome
We developed a COVID-19 severity index using the information on admission and discharge units, length of stay, indications of ventilation, and indications of mortality. This severity index had 5 non-overlapping, independent levels. These levels, from least severe to most severe, were as follows: negative SARS-CoV-2 test, positive diagnostic test and outpatient care (outpatient), positive diagnostic test and emergency department (ED) care, positive diagnostic test and hospitalization (hospitalization), and positive diagnostic test and in-hospital mortality (death). A novel aspect of this severity index includes outpatient and ED care. Outcome categorization heavily relied on patterns in admission and discharge units and indications of ventilation and mortality. However, when these were unknown, illness severity assignment was made using responses in clinical variables. Individuals were sorted based on the highest level of care received. For example, individuals who were first tested in the ED and subsequently hospitalized were categorized as hospitalized. The individuals first tested in an outpatient setting who later died while under hospital care were classified as in-hospital death, and so on.
Inclusion and Exclusion
Individuals who tested positive but did so while seeking care for another condition (e.g., pre-surgery testing) were removed from the sample, as our objective for this study was to evaluate COVID-19 severity among individuals seeking care due to the virus. Basic information on admission, disposition, or patient type was excluded from the analysis. It should be noted that all individuals who tested negative for the virus were included only in the descriptive analysis and were removed during inferential statistics. These individuals who tested negative were also removed during the length of stay analysis. Similarly, those who identified as non-binary or did not disclose their gender were removed from inferential analysis due to low frequency.
Statistical Methods
The associations between sociodemographic characteristics and COVID-19 illness severity were analyzed using standard cross-tabulation methods, with all categorical results reported in frequencies and percentages. Length of stay for all severity levels was characterized using medians, interquartile ranges, and ranges. We used multivariable logistic regression to generate odds ratios (95% CI) on associations between race, ethnicity, and gender and the likelihood of ED and inpatient hospitalization care. Reference categories for gender, age, and race and ethnicity were as follows: female, 18 to 25 years of age, and WNH. Model fit was assessed using Akaike Information Criterion (AIC). Adjusted multinomial logistic regression was employed to assess demographic predictors of COVID-19 death using the same reference categories for gender, age, and race and ethnicity. We report missing values for our predictors and outcomes. All models were a complete case analysis. We used Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC) for all analyses.
Results
Characterization of Sample
Of the 70,913 tested for SARS-CoV-2 from March 10, 2020, to July 10, 2020, 6470 adults tested positive for SARS-CoV-2, and 6445 were eligible for this study. Of the 6445 individuals eligible for this study, race and ethnicity were known for 4829 (85.8%), gender identity was known for 6424 (83%), and age was known for all (100%). There were 25 deaths before discharge, leaving a sample of 6420 for the multivariable analyses. Just over half of the sample population identified as female (53.3%). 12.6 percent of the sample population was 65 years or older during testing. Finally, most individuals in this sample identified as WNH (61.0%) (Table 1).
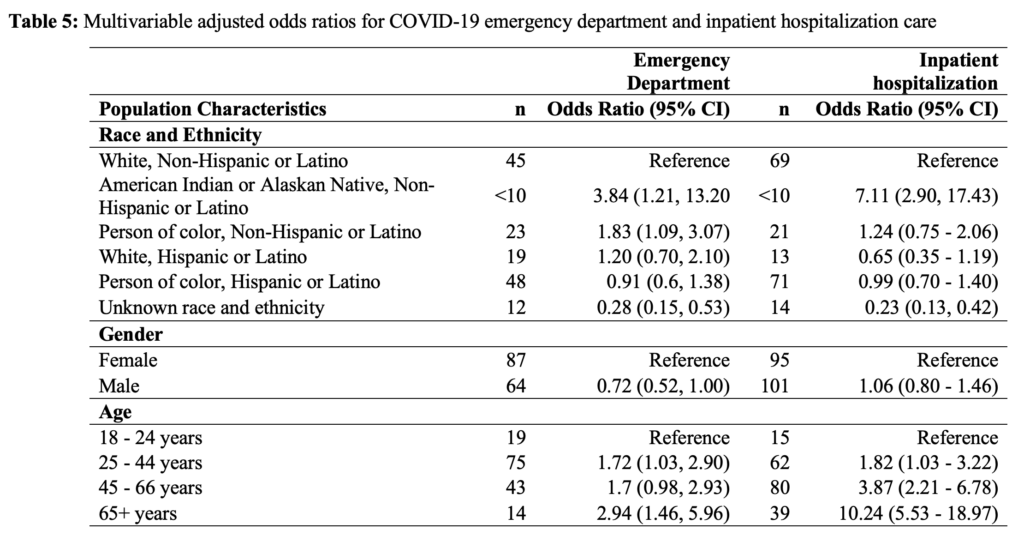
An overall percent positivity of 9.1 percent was observed in this population between March 10, 2020, and July 10, 2020. Notably, all non-White individuals (23.1%) exhibited a proportionally higher percent positivity than WNH individuals (3.9%). Individuals who identified as female had a slightly lower percent positivity than those who identified as male (8.2%, 10.1%, respectively). Individuals who identified as non-binary or did not disclose their gender had the highest percent positivity of the strata (16.9%) (Table 2).
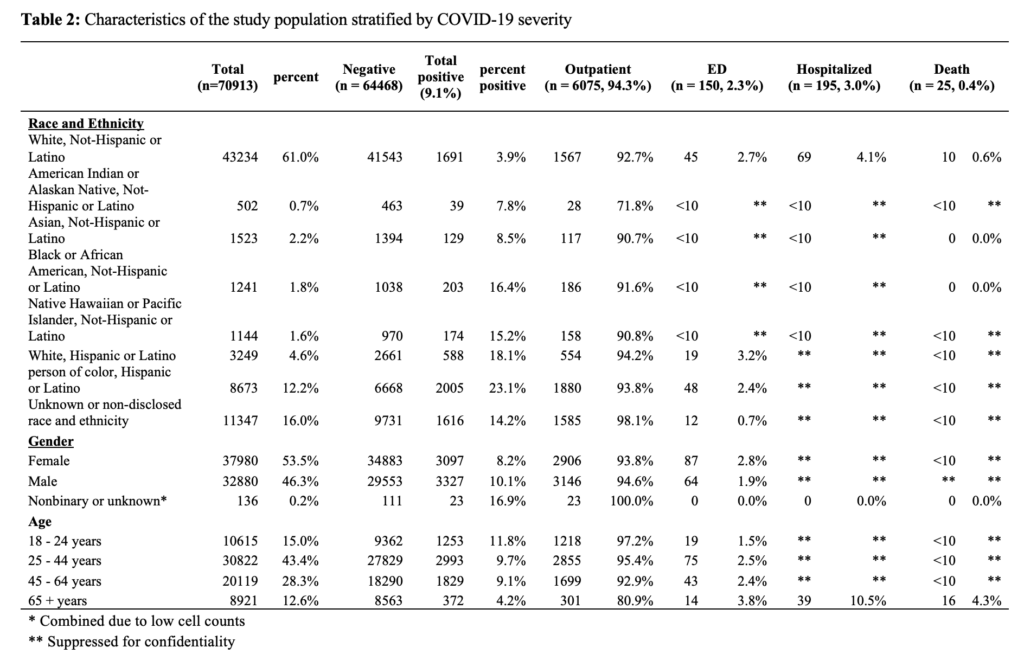
Out of the 6445 individuals who tested positive for SARS-CoV-2, 6075 (94.3%) received outpatient care, 150 (2.3%) received care in the emergency department, 195 (3.0%) were hospitalized, and 25 (0.04%) individuals died in hospital (Table 3).
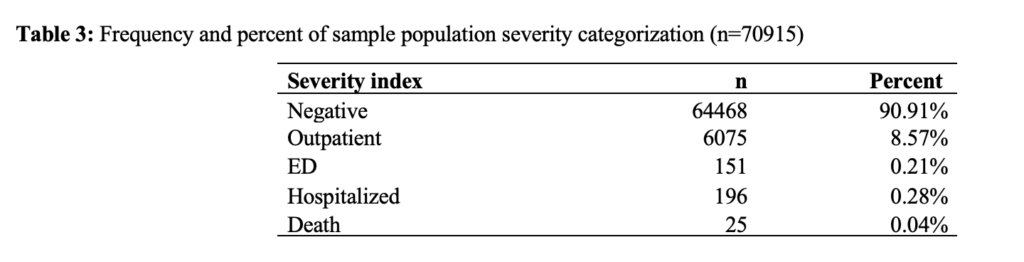
Length of stay in care by type of care for this study population was as follows: outpatient (median=13 hours, IQR=4 hours), emergency department (median=4 hours, IQR=7 hours), inpatient hospitalization (median=52 hours, IQR=98 hours), patients requiring ventilation (median=285 hours, IQR=671), and those patients who died in hospital (133 hours, IQR=186) (Table 4).
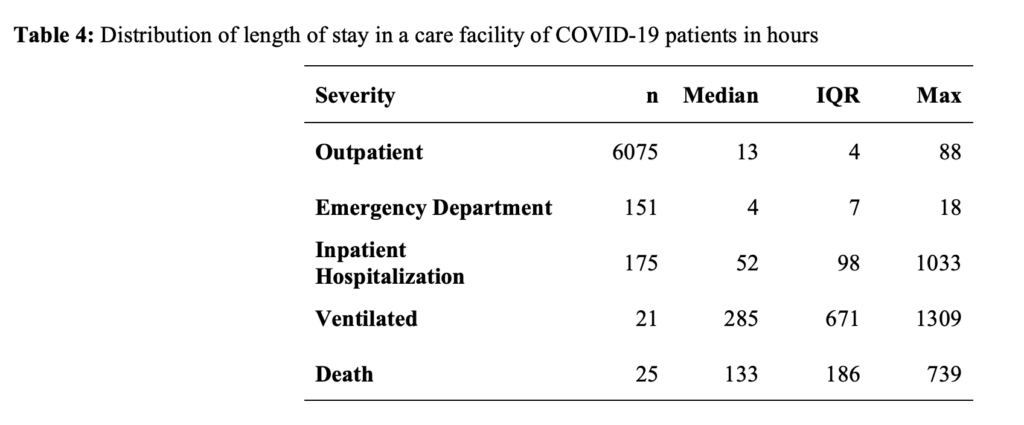
Compared to WNH, racial and ethnic minoritized groups had increased adjusted odds ratios (aOR) of receiving emergency department care or hospitalization (aOR =1.83 [95% CI: 1.09, 3.07]; aOR = 7.11 [95% CI: 2.90, 17.43], respectively) (Table 5). Compared to patients younger than 65 years, patients 65 years or older had increased odds of receiving emergency department care or hospitalization (aOR =2.94 [95% CI: 1.46, 5.96]; aOR = 10.24 [95% CI: 5.53, 18.97], respectively). Compared to women, men had similar odds of receiving emergency department care or hospitalization (aOR = 0.72 [95% CI: 0.52, 1.0] and aOR = 1.06 [95% CI: 0.80, 1.46], respectively).
Discussion
In this study, we aimed to describe differences in COVID-19 outcome severity by race, ethnicity, gender identity, and age. We found that among the 9.1 percent (n = 6,420) of patients who tested positive for SARS-CoV-2 at University of Utah Health facilities from March to July of 2020, 95 percent received outpatient care, approximately 2 percent received care in the emergency department, 3 percent were hospitalized, and 0.4 percent died. Differences in COVID-19 illness severity could be partially explained by racial identity. Individuals who identified as “American Indian or Alaskan Native, non-Hispanic or Latino,” or “person of color, non-Hispanic or Latino” had higher odds of receiving ED care than WNH individuals. Similarly, “American Indian or Alaskan Native, non-Hispanic or Latino” individuals had higher odds of being hospitalized than WNH individuals.
This study’s results are generally in line with prior research reporting an increased level of COVID-19 illness severity in non-White individuals compared to persons of color.23 Similarly, work utilizing the Coronavirus Disease 2019-Associated Hospitalization Surveillance Network (COVID-NET) found that the highest hospitalization, hospitalization in intensive care units (ICU), and death rates occurred among Hispanic or Latino individuals and Black or African American individuals.16,24 Our results deviate slightly from previous work in that associations between being White, Hispanic or Latino, versus WNH, and COVID-19 illness severity were largely null.
Our null findings could be due to several reasons. First, it is possible that ethnic identity between these groups did not explain the likelihood of severe COVID-19 outcomes in this population during the pandemic. Second, immigration status in comorbidity burden may play a significant role in illness outcome but were not considered in this current work. Finally, we had limited power to detect differences within our early pandemic sample. Larger studies within Utah are needed before definitive conclusions can be made.
Individuals who did not disclose their race and ethnicity or were documented as being of an unknown race or unknown ethnicity had lower odds of receiving ED care or being hospitalized. This is not due to inherent protection inferred by non-disclosure of race and ethnicity but due to an evident reporting bias: it appears that patients are less likely to disclose their race and ethnicity when receiving care in the outpatient setting than patients who require higher levels of care (eg, ED or hospitalization). This also indicates individuals’ lack of trust when seeking healthcare for non-critical situations.
Depending on social and biological determinants, disease experience and burden vary in different groups. The COVID-19 pandemic and associated diseases are no different and have highlighted and broadened persistent health disparities in the United States.23, 25, 26 Racial and ethnic minoritized groups are more likely to reside and work in high transmission areas, have less access to testing and vaccination services, and present with a higher level of illness severity when seeking care. Additionally, racial and ethnic minoritized groups are, in general, more burdened by comorbidities that are strongly associated with severe COVID-19 outcomes.27 Notably, previous work in Utah calls attention to the fact that many areas with high health disparities have proportionally larger racial and ethnic minoritized populations than areas with low health disparities.27 This work suggests that there may be an association between compounded transmission and racial and ethnic minoritized individuals residing in Utah.16,23,24
Limitations and Strengths
This study is subject to 2 distinct limitations. First, this study only captures illness severity through the lens of clinical outcomes in patients who sought testing and treatment at 1 medical network during the pandemic’s beginning. As such, these results may not be generalizable to all COVID-19 patient experiences across the pandemic, characterized by various surges of different SARS-CoV-2 lineages, dissemination of vaccines and treatments, and changes in public health response. Secondly, this study did not account for all structural and social determinants that have been identified as affecting health outcomes (socioeconomic status, insurance coverage, occupation, place of residence, level of educational attainment, etc.). Consequently, these results do not capture all variations in COVID-19 illness severity influenced by structural and social determinants.
However, this work has its strengths. First, we describe and characterize illness severity using a granular index. This is distinct, as most work early in the pandemic assessed illness severity using only 3 levels of severity rather than the 5 developed for this study. Secondly, this work captured information on race and ethnicity for 84 percent of individuals in the sample. This is notable as race and ethnicity are often under-reported in medical and public reports. Finally, while this work only focuses on the earliest stage of the COVID-19 pandemic, it sets a solid methodological foundation for future infectious disease studies in Utah using the Enterprise Data Warehouse, especially those designed to capture illness severity.
Conclusion
This retrospective descriptive analysis found that COVID-19 illness severity, measured by an expanded illness severity index, varies by race, ethnicity, and age. In Utah, the likelihood of more significant illness severity outcomes disproportionately impacts older individuals and individuals identifying as “American Indian or Alaskan Native and non-Hispanic or Latino” or “person of color and non-Hispanic or Latino.” Further work is needed to understand the complexities of the COVID-19 disease experience and how it has changed over time in Utah and throughout the Intermountain West. Specifically, an emphasis on structural and social determinants of COVID-19 illness, while controlling for biological determinants, across the entire pandemic is needed.
Acknowledgements
We are grateful for healthcare professionals working tirelessly to combat the COVID-19 pandemic. The University of Utah Electronic Data Warehouse provided data for this study.
References
1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
2. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi:10.1186/s40249-020-00646-x
3. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. Accessed April 15, 2022. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
4. Centers for Disease Control and Prevention. CDC COVID Data Tracker: Home. Accessed April 15, 2023. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
5. Selden TM, Berdahl TA. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Affairs. 2020;39(9):1624-1632. doi:10.1377/hlthaff.2020.00897
6. Martinez DA, Hinson JS, Klein EY, et al. SARS-CoV-2 Positivity rate for Latinos in the Baltimore-Washington, DC region. JAMA. 2020;324(4):392-395. doi:10.1001/jama.2020.11374
7. Karaye IM, Horney JA. The impact of social vulnerability on COVID-19 in the US: An analysis of spatially varying relationships. American Journal of Preventive Medicine. 2020;59(3):317-325. doi:10.1016/J.AMEPRE.2020.06.006/ATTACHMENT/5E18ADFE-2EA7-4E86-B70D-329794452EEA/MMC1.PDF
8. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on Black communities. Annals of Epidemiology. 2020;47:37-44. doi:10.1016/J.ANNEPIDEM.2020.05.003
9. Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. American Journal of Industrial Medicine. 2020;63(9):817-820. doi:10.1002/AJIM.23145
10. Rader B, Astley CM, Sy KTL, et al. Geographic access to United States SARS-CoV-2 testing sites highlights healthcare disparities and may bias transmission estimates. Journal of Travel Medicine. 2020;27(7):taaa076. doi:10.1093/jtm/taaa076
11. Simmons A, Chappel A, Kolbe AR, Bush L, Sommers BD. Health disparities by race and ethnicity during the COVID-19 pandemic: Current evidence and policy approaches. ISSUE BRIEF. 2021;1. Accessed April 18, 2022. https://www.cdc.gov/nchs/nvss/vsrr/COVID19/index.htm
12. Vahidy FS, Nicolas JC, Meeks JR, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: Analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8):e039849-e039849. doi:10.1136/bmjopen-2020-039849
13. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Affairs. 2020;39(7):1253-1262. doi:10.1377/hlthaff.2020.00598
14. Adegunsoye A, Ventura IB, Liarski VM. Association of Black race with outcomes in COVID-19 disease: A retrospective cohort study. Ann Am Thorac Soc. 2020;17(10):1336-1339. doi:10.1513/AnnalsATS.202006-583RL
15. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19—United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. doi:10.15585/MMWR.MM6942E1
16. Hsu HE, Ashe EM, Silverstein M, et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an Urban Safety-Net Medical Center—Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):864-869. doi:10.15585/MMWR.MM6927A3
17. Strully K, Yang TC, Liu H. Regional variation in COVID-19 disparities: Connections with immigrant and Latinx communities in US counties. Ann Epidemiol. 2021;53:56-62.e2. doi:10.1016/j.annepidem.2020.08.016
18. Berchick ER, Hood E, Barnett JC. Health Insurance Coverage in the United States: 2018. Washington, DC: US Department of Commerce; 2019.
19. Acosta AM, Garg S, Pham H, et al. Racial and ethnic disparities in rates of COVID-19–Associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to February 2021. JAMA Network Open. 2021;4(10):e2130479-e2130479. doi:10.1001/jamanetworkopen.2021.30479
20. US Census Bureau. US Census Bureau QuickFacts: United States. Accessed April 15, 2022. https://www.census.gov/quickfacts/fact/table/US/PST045219.
21. About University of Utah Hospitals & Clinics. University of Utah Health. Accessed April 16, 2022. https://healthcare.utah.edu/about/
22. Dalsania AK, Fastiggi MJ, Kahlam A, et al. The relationship between social determinants of health and racial disparities in COVID-19 mortality. Journal of Racial and Ethnic Health Disparities. 2022;9(1):288-295. doi:10.1007/s40615-020-00952-y
23. Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: A systematic review and meta-analysis. JAMA Network Open. 2021;4(11):e2134147-e2134147. doi:10.1001/jamanetworkopen.2021.34147
24. Mein SA. COVID-19 and health disparities: The reality of “the Great Equalizer.” Journal of General Internal Medicine. 2020;35(8):2439-2440. doi:10.1007/s11606-020-05880-5
25. Killerby ME, Link-Gelles R, Haight SC, et al. Morbidity and mortality weekly report characteristics associated with hospitalization among patients with COVID-19–Metropolitan Atlanta, Georgia, March-April 2020. Accessed April 15, 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-
26. Moore JT, Pilkington W, Kumar D. Diseases with health disparities as drivers of COVID-19 outcome. Journal of Cellular and Molecular Medicine. 2020;24(19):11038-11045. doi:https://doi.org/10.1111/jcmm.15599
27. Lewis NM, Friedrichs M, Wagstaff S, et al. Disparities in COVID-19 incidence, hospitalizations, and testing, by area-level deprivation—Utah, March 3–July 9, 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69(38):1369-1373. doi:10.15585/MMWR.MM6938
Citation
Paegle AJ, VanDerslice JA, Benson LS, Schliep KC. (2023). Socio-Demographic Disparities in SARS-CoV-2 Illness Severity; Utah, 2020. Utah Women’s Health Review. doi: 10.26054/0d-7tf3-0ah7.

