It has long been recognized that optimal pregnancy outcomes, namely an uncomplicated full-term vaginal delivery for mother of a normal healthy child, are most likely to occur in the maternal age decade from late teens through late twenties. It is not by accident that this is also the decade at which humans are at their cardiovascular and musculoskeletal prime. Women in this age window are optimally likely to tolerate the multiple physiologic changes that occur during pregnancy. The placenta, which is a fetal structure, secretes large quantities of hormones into the mother’s bloodstream, which produce these maternal adaptations (Table 1).

Although most pregnant women successfully adapt to these changes, serious complications for mother and child still occur. These complications are more common when co-existing maternal medical complications are present and when maternal age gets further away from the optimal window.
The current paradigms for prenatal care aim to identify those pregnancies at increased risk for serious pregnancy complications such as stillbirth, premature delivery, preeclampsia/eclampsia, or life-threatening hemorrhage. This concept of risk prediction is an established and accepted practice in clinical medicine and is now widely utilized in efforts to reduce the burden of the chronic non-infectious diseases of adulthood such as diabetes, hypertension, heart disease, and kidney failure. A pioneering contributor to this concept is the Framingham Heart Study. Launched in 1948, the study has demonstrated the utility of clinical and laboratory findings to identify common factors or characteristics that contribute to heart disease and has evolved into a multigenerational study of family patterns of cardiovascular and other diseases.1 An important further contribution of the Framingham Study has been its successful efforts to follow a large cohort of individuals, and now their descendants, across multiple decades to confirm the association of their initial data from decades before with the presence or absence of subsequent chronic disease. Given the complex natures of modern societies and the generally fragmented nature of healthcare systems, such long-term prospective follow-up studies remain challenging but uniquely valuable.
Several other early landmark studies have helped focus our current attention on pregnancy as a window to the future health of both the mother and her offspring. In 1964, Mahan and O’Sullivan described the association of abnormal glucose metabolism in pregnancy with the subsequent development of diabetes over the ensuing eight years.2 Of historical interest, this manuscript provided no information on pregnancy outcomes or complications. Perinatal complications with gestational diabetes were only described in detail in the following decades.3
In the 1980s, David Barker began a series of studies looking at the outcomes of children born of pregnancies complicated by low birth weight and found higher incidence of ischemic heart disease in surviving children.4 The subsequent “Barker hypothesis” is an association between fetal adaptations to placental insufficiency and subsequent chronic diseases of adulthood.
These studies, and others, have led to an increasing recognition that pregnancy complications identify a subset of women and children at increased risk for long-term adverse health outcomes. With this increasing recognition of the long-term effects of pregnancy complications, optimal strategies to screen women at-risk for adverse health outcomes throughout adulthood are the subject of active investigation. Below is a description of health-related issues that can serve as the windows to women’s future health.
Gestational Diabetes (GDM)
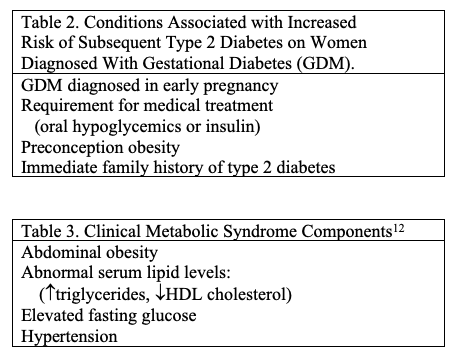
As mentioned, pregnant women who develop gestational diabetes are at increased risk for type 2 diabetes. Women who are diagnosed with GDM early in pregnancy, who require medical treatment, who are obese, or have an immediate family history of type 2 diabetes are at higher risk for this complication (Table 2). Many of these women will develop overt diabetes within the first five years after delivery, at least in part because some of them had undiagnosed type 2 diabetes preconception. This issue emphasizes the importance of periodic postpartum blood glucose assessment for women with GDM, particularly if they have additional risk factors (Table 3). Women with GDM who also have other components of the metabolic syndrome are at additional increased risk for subsequent cardiovascular disease (Table 3).
Hypertensive Disorders of Pregnancy (HDP)
Hypertensive disorders of pregnancy, defined as gestational hypertension (BP > 140/90 mmHg without proteinuria), pre-eclampsia (BP >140/90 mmHg plus proteinuria, with or without other laboratory abnormalities or symptoms), or eclampsia (seizures in a pre-eclamptic woman without other obvious cause), are common pregnancy complications, complicating about one in eight (13.0%) pregnancies in the United States.5 HDP are more common in non-Caucasian, older (esp. age >35), and obese women. Women with any history of HDP are at increased risk for cardiovascular disease and type 2 diabetes later in life, presumably because the physiologic stresses of pregnancy unmask the underlying predisposition many of these individuals have for subsequent microvascular disease(s). There is also evolving evidence that a history of HDP predisposes to dementia, particularly vascular dementia, later in life.6 Thus, women with any history of HDP, but particularly those with the aforementioned risk factors and/or HDP in more than one pregnancy, are candidates for early screening for evidence of evolving or progressive microvascular disease (Table 3).
Preterm Birth (PTB)
Preterm birth, defined as delivery prior to 37 weeks gestation, may be either spontaneous or indicated. Medically indicated PTB may be the result of either maternal or fetal indications but is generally undertaken at such point in pregnancy as either the mother is better off not being pregnant or the fetus can be expected to fare as well, or better, in the (intensive care) nursery than in utero. These two scenarios cover a broad range of maternal and fetal/placental conditions and complications. Many of these conditions are associated with systemic inflammation and vascular senescence. These same underlying mechanisms have also been associated with spontaneous preterm birth and with early cardiovascular disease.7,8
Fetal Growth Restriction (FGR)
Fetal growth restriction, frequently defined as the lowest decile of birthweights for a given gestational age and fetal sex, can result from maternal medical conditions, placental dysfunction, or intrinsic fetal abnormalities. The “Barker Hypothesis” mentioned above generated multiple subsequent observational studies that confirmed the increased risk of adult-onset microvascular diseases for both the child and the mother.4
Infertility
The previous conditions all reflect an increased risk of subsequent microvascular disease in women who suffer various pregnancy complications. It should be noted that the inability to achieve a wanted pregnancy (infertility) has also been associated with an increased risk of cardiovascular disease.9,10 Much of this association is mediated by the presence of polycystic ovarian syndrome (PCOS), the most common cause of infertility in women. Of note, in addition to increased risks of cardiovascular disease, women with infertility related to PCOS are at elevated risk for endometrial cancer.11
Table of Contents
Fetal Origins of Adult Disease
Another impact on women’s health in later life is linked to the fetal origins of adult disease. While this section is focused on long-term maternal health consequences of pregnancy complications, it must be emphasized that children born of these pregnancies are also at increased risk of non-infectious chronic diseases of adulthood. These risks are due in part to genetic predispositions but are also enhanced by the in utero environment associated with these pregnancy complications. This concern is widely referred to as epigenetic influences.
Management Strategies for Women Following Pregnancy Complications
Pre-conceptional obesity increases the risk for the aforementioned pregnancy complications (and also for infertility). Postpartum weight reduction programs (diet, exercise) should emphasize the benefits of weight reduction for decreasing the risks of subsequent diabetes and hypertension. That said, sustained weight loss is both difficult to achieve and to maintain. Consideration of a household-focused lifestyle change may well be required. In refractory cases, especially in the presence of additional co-morbid conditions, medical and surgical weight management interventions may be considered. Additionally, if these women are smokers, they should be provided with every option to stop. Women with these concerns should be particularly encouraged to breastfeed their infants. Breastfeeding, particularly if exclusive and for at least six months, can decrease cardiovascular risks both for mothers and for their children.
In addition, women who have experienced these pregnancy complications should be followed for pre-symptomatic evidence of evolving microvascular disease. As mentioned, many women who develop GDM will become overtly diabetic within a few years after delivery. Likewise, women who experience HDP are more likely to have evidence of underlying microvascular disease even within a few years following delivery. Identification of these risk factors can allow early intervention that can improve these individuals’ health over their subsequent lifespan.
Acknowledgements
Dr. Theilen is supported in part by NIH grant K23 HL159316. Dr. Varner is supported in part by NIH grant K12 TR004413.
References
- Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999-1008. doi:10.1016/S0140-6736(13)61752-3
- O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278-285.
- Langer O, Mazze R. The relationship between large-for-gestational-age infants and glycemic control in women with gestational diabetes. Am J Obstet Gynecol. 1988;159(6):1478-1483. doi:10.1016/0002-9378(88)90578-9
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077-1081. doi:10.1016/s0140-6736(86)91340-1
- Ford ND, Cox S, Ko JY, et al. Hypertensive Disorders in Pregnancy and Mortality at Delivery Hospitalization – United States, 2017-2019. MMWR Morb Mortal Wkly Rep. 2022;71(17):585-591. Published 2022 Apr 29. doi:10.15585/mmwr.mm7117a1
- Schliep KC, Mclean H, Yan B, Qeadan F, Theilen LH, de Havenon A, Majersik JJ, Østbye T, Sharma S, Varner MW. Association Between Hypertensive Disorders of Pregnancy and Dementia: a Systematic Review and Meta-Analysis. Hypertension. 2023 Feb;80(2):257-267. doi: 10.1161/HYPERTENSIONAHA.122.19399. Epub 2022 Nov 8. PMID: 36345823; PMCID: PMC9851987.
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760-765. doi:10.1126/science.1251816
- Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Ann Epidemiol. 2015 Jan;25(1):40-5. doi: 10.1016/j.annepidem.2014.10.012. Epub 2014 Oct 18. PMID: 25459086; PMCID: PMC4406400.
- Udell JA, Lu H, Redelmeier DA. Failure of fertility therapy and subsequent adverse cardiovascular events. CMAJ. 2017;189(10):E391-E397. doi:10.1503/cmaj.160744
- Dayan N, Filion KB, Okano M, et al. Cardiovascular Risk Following Fertility Therapy: Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2017;70(10):1203-1213. doi:10.1016/j.jacc.2017.07.753
- Parikh NI, Jeppson RP, Berger JS, et al. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation. 2016;133(22):2149-2158. doi:10.1161/CIRCULATIONAHA.115.017854
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237. doi:10.1242/dmm.001180
Citation
Adebayo A, Theilen L, & Varner M. (2024). Pregnancy as a Window to Women’s Future Health: An Essay. Utah Women’s Health Review.

